Today we are pleased to welcome Dr. Majid Afshar, one of the newest members of the Division of Pulmonary & Critical Care Medicine at the University of Maryland. Today he was willing to share his tips and tricks to sorting out the handful of good research among the thousands of paper released every year. In the next 45 minutes he will take you from entry level novice to epidemiology expert!
Podcast: Play in new window | Download
Subscribe: Apple Podcasts | RSS
Pearls
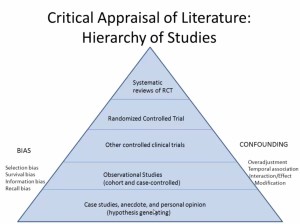
Internal Validity
- Error Types:
- Random Error: depends on chance alone (ex: flipping 10 coins)
- Decreases as sample size increases
- Systemic Error or Bias
- Survival Bias/Incidence Prevalence Bias: selective survival among the prevalent cases
- Loss to Follow-up Bias
- Publication Bias: only publish positive studies
- Information Bias
- Misclassification Bias: ex: Recall Bias, Observer Bias, Interviewer Bias, Dilution Bias
- Lead Time Bias: early diagnosis falsely appears to prolong survival
- Random Error: depends on chance alone (ex: flipping 10 coins)
- Confounding
- An apparent association between disease and exposure caused by a third factor not taken into consideration
- Can be avoided with a RCT or with Matching
External Validity
- Measures of Effect
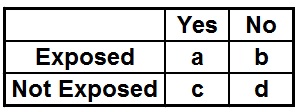
- Control event rate (CER) = c/c+d
- Experimental event rate (EER) = a/a+b
- Relative Risk (RR) = EER/CER=(a/a+b)/(c/c+d)
- Relative Risk Reduction (RRR) = CER-EER/CER
- commonest reported measure of dichotomous treatment effect
- Absolute Risk Reduction (ARR) = CER-EER
- Number Needed to Treat (NNT) = 1/ARR
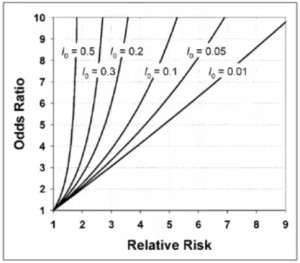
- Odds Ratio (OR) = a*d/c*b
- OR estimates the RR ONLY if disease is rare
- Composite Outcome
- Combine a number of individual outcomes (such as types of morbidity)
- Used when no obvious choice of primary endpoint
- Allows for a smaller sample size and/or a shorter follow-up
- However: difficult to use and interpret, leading to errors in sample size estimation, possible contradictory trial results, and difficulty in interpreting findings.
- Subgroup Analysis
- Evaluate treatment effects and/or specific outcomes in subgroups of patients
- Often difficult to interpret, as subgroups are often only identified after the fact (not a priori)
References
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63.
- Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 2002;347:1143-1150.
- Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988 Aug 13;2(8607):349-60.