Today we are honored and privileged to have one of the premier minds in the field of critical care epidemiology, Dr. Burton Lee. In addition to his role as program director of the Pulmonary Critical Care & Emergency Medicine Critical Care Fellowship at the MedStar Washington Hospital Center, Dr. Lee spends a significant amount of time transforming the most complex statistical analysis techniques into such simplistic terms that even a novice can fully comprehend. Today we learn the tips and tricks to tackle numeracy and the α-error, 2 topics that you THINK you know, but do you really get it?!?
Podcast: Play in new window | Download
Subscribe: Apple Podcasts | RSS
Pearls:
- Numeracy: mathematical competence, ie: applying simple concepts of mathematics to reason
- Apophenia: seeing patterns in randomness/meaningless data (example: pictures in the clouds)
- Type I error or α-error: conclude a “pattern” or “result” when data is truly random
- Type II error or β-error: incorrectly conclude no pattern exists
- Type I error or α-error: conclude a “pattern” or “result” when data is truly random
- In order to be an astute reader of the medical literature you must understand the pitfalls of four commonly used statistical methods:
- Multiple Testing: with multiple testing of the same concept, eventually you will get a positive study (by chance alone)
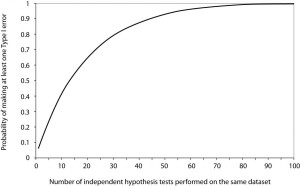
-
- Will see an eventual elimination of α-error
- Outcome changes: fitting the outcome of a study to a more publishable, “positive outcome”
- Lessens adherence to α-error and hurts the overall power of the study
- Can be remedied with preregistered outcomes: http://clinicaltrials.gov/
- Publication bias: publication of only studies that support a desired outcome
- Turner. NEJM 2008;358:252-60
- The only solution is to report ALL clinical trials, again reliance on http://clinicaltrials.gov
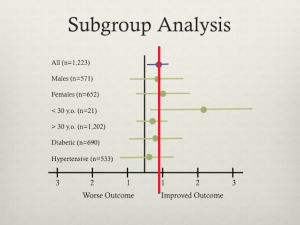
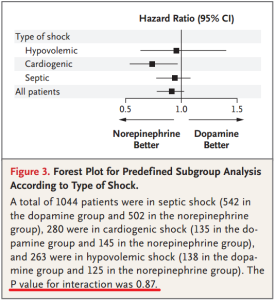
- Subgroup analysis: making conclusions by post-hoc analysis of randomly chosen subgroups
- ISIS-2. Lancet 1988;ii:349-60
- Remedied with pre-specification of subgroups prior to experimentation (not meant to replace hypothesis generation)
- Criticize existing subgroup analysis by 2 methods:
- Multiple Testing: with multiple testing of the same concept, eventually you will get a positive study (by chance alone)
De Backer. NEJM 2010;362:779-789
References
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA. 2009 Sep 2;302(9):977-84.
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008 Jan 17;358(3):252-60.
- Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature 2009;483:531–533.