Today we welcome Dr. Thomas Pallone, Professor of Medicine in the Division of Nephrology here at the University of Maryland. In addition to being a brilliant clinician, Dr. Pallone also spent time doing research at the Medical Engineering Medical Sciences division of the HST program at the Massachusetts Institute of Technology. It is safe to say, he is one of the most brilliant minds we have at Maryland and today he takes an hour to give us a simplified look at the way the kidneys deal with acids and bases. This talk will give you a common sense look at renal physiology and just might give you the information you need to crack the next complex renal failure case!
Clinical Pearls
- Catabolism normally creates:
- 20 moles of CO2/day (0.88kg/day) → lungs
- ~70 mMoles strong acids/day → kidneys
- Fate of an ECF proton:
- RBCs are an important part of the buffering process, speeds reaction with carbonic anhydrase (CA)
- Works well to exchange the anions/cations via the membrane
- The glomeruli filters HCO3
- 25 mEq/L for 180L/day = 4,500 mEq/day!
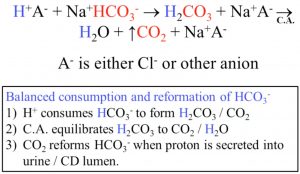
- Reclaimed in proximal convoluted tubule (PCT) via gradient flow using CA and acidification = breaks even
- HCO3 is replaced in the collecting duct (CD) + PCT
- Excretes protons into the urine that is absorbed by the buffers (TA’s, NH3/NH4)
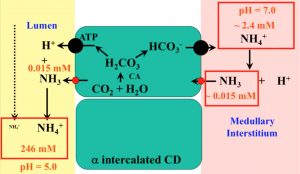
- NH3 → NH4 (a H+ sink!) with a pKa of 9.2 (or 100 to 10,000 more NH4>NH3)
- Changes in pH makes HUGE swings to this ratio (159 at pH 7 → 15,873 at pH 5!)
- NH3 → NH4 (a H+ sink!) with a pKa of 9.2 (or 100 to 10,000 more NH4>NH3)
- Excretes protons into the urine that is absorbed by the buffers (TA’s, NH3/NH4)
- PCT can produce new HCO3 from glutamine, which creates 2 new bicarb ions (into blood stream) and an ammonium ion (which is excreted to the lumen)
- NH4 is reabsorbed by the loop of Henle → to be secreted by the CD (cortico-med gradient NH4/NH3/diffusion trapping)
- 25 mEq/L for 180L/day = 4,500 mEq/day!
- High NH4 can thus be excreted w/o a significant osmolar presence of NH4 in the medullary interstitium
- The difference in NH4 btw the CD lumen and medullary interstitium increases 1 order of magnitude for every 1.0 pH difference!
Suggested Reading
- Wagner CA, Bourgeois S. Two Rhesus protein ammonia transporters team up to eliminate ammonium into urine. Am J Physiol Renal Physiol. 2014 Apr 1;306(7):F721-3. [PubMed Link]
- Berend K, de Vries AP, Gans RO. Physiological approach to assessment of acid-base disturbances. N Engl J Med. 2014 Oct 9;371(15):1434-45. [PubMed Link]